In recent weeks, many companies have announced their plans to replace conventional lithium-ion batteries with a new technology called solid-state batteries. This article gives you a brief overview on what these new batteries are and how they solve the problems that conventional lithium-ion batteries pose.
Source: QuantumScape
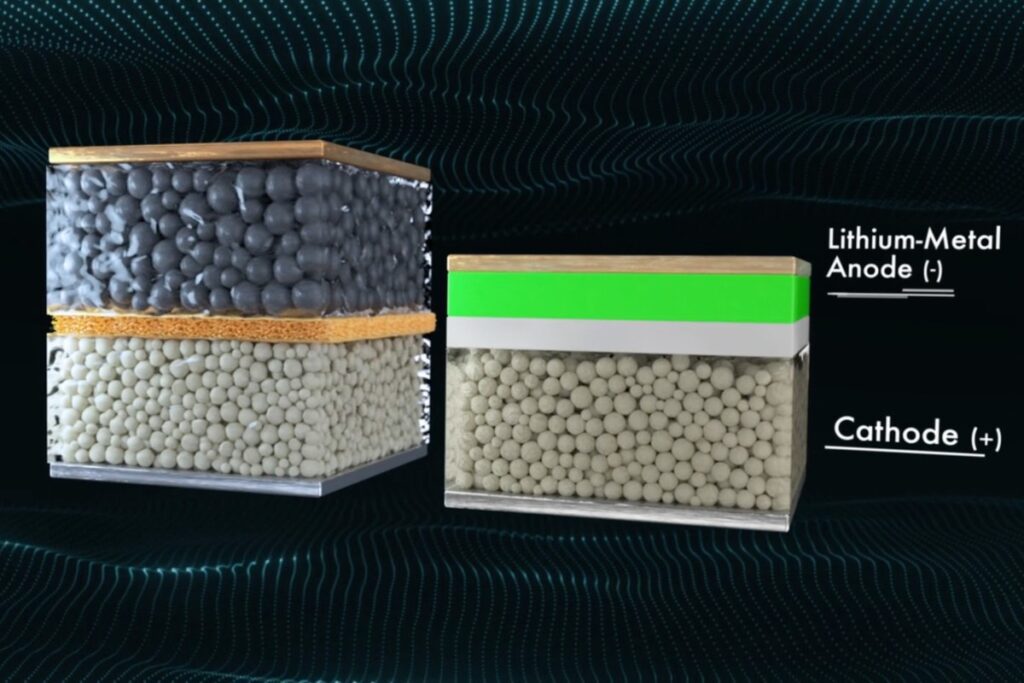
In conventional batteries, the anode and the cathode are isolated by a separator. This thin layer of polymer or ceramics is permeable for the lithium-ions. For them to be able to move freely from anode to cathode during discharge (and the other way around while charging), the battery has to be filled with a conductive fluid, the electrolyte.
In solid-state batteries (SSB), this electrolyte is – as the name implies – solid. There are three categories of solid electrolytes: polymers, oxides and sulfides. Its not yet clear which substance is best. Conventional batteries use graphite as a host matrix for the lithium-ions at the anode. Many SSB feature either an anode-free design or a lithium metal anode.
To be clear – SSB also function on the basis of lithium-ions moving through the cell, but they are not called lithium-ion batteries, this name mostly refers to batteries with a fluid electrolyte. If you want to see a more detailed depiction on how these batteries work, I recommend this video by Quantumscape.
SSB might solve some of the problems that the use of lithium-ion batteries poses. Some benefits include:
- They are not flammable and there are no volatile substances that are released when a cell is damaged. This could make the batteries more safe, depending on the reactivity of the other materials that are used.
- In conventional cells there is a layer called the Solid Electrolyte Interphase (SEI), that forms between the anode and the liquid electrolyte during the first charging cycles. This so-called passivation layer shortens the life span of the cell. To minimize this effect, the SEI should be as uniform as possible. That’s why the first couple of charging cycles are performed in the factory under ideal conditions (formation). Since there is no SEI that forms in solid-state batteries, this loss of capacity can be avoided.
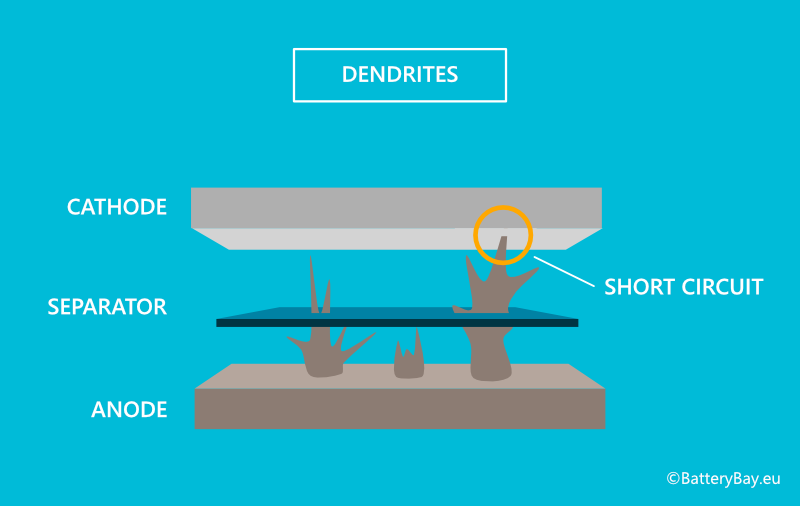
- During the lifespan of lithium-ion batteries, the lithium can grow into tree-like shapes called dendrites. These outgrowths can puncture the separator which can cause the cell to short circuit and become unusable. This is very common with SSB. However, the solid electrolyte can inhibit further dendrite growth and protect the separator from being damaged.
- Solid-state batteries are tolerant to overcharging. Overcharging conventional batteries causes the liquid electrolyte to overheat and to release gases, which diminishes the battery’s capacity in the long run.
- Some solid-state batteries can withstand extreme temperatures. Conventional batteries can lose capacity when exposed to very high or low temperatures.
By solving these problems and by promising higher energy densities as well as faster charging speeds, SSB will become a serious competitor for the batteries developed by Tesla, CATL and Panasonic. However, the price of solid-state batteries still exudes the cost of conventional battery cells. No company has been able to produce solid-state batteries for EV applications at scale. It will take a couple more years to see when or whether solid-state batteries will replace the current battery technology.
If you want to know how different companies try to approach these issues, I recommend my article “The State of Solid-State“.
Connect with me on Twitter (@BatteryBayEU) to keep up to date on the battery ecosystem. I’m looking forward to learning about your involvement or interest in the industry and chatting about everything batteries.
Also, please feel free to use the comment section below to leave any feedback or suggestions!
Keep up the nice work, Joanna!
Just a feew things that caught my attention and that you could check on:
1. Dendrite growth usually refers to Li-Dendrites, i.e. as soon as Li plating started at some spot, this will be the preferred spot for further Li deposition and a dendrite starts growing. A thick SEI will forward Li Plating, but I am not sure if the SEI really forms dendrite-shapes itself.
2. I think dendrite growth does indeed also occurs in ASSB. Especially when going all-in and using a Li-metal anode.
3. The temperature performance strongly depends on the solid electrolyte that is used – some only allow sufficient Li-ion transport at elevated temperatures. So be careful not to trivialize the temperature influence.
4. Regarding safety: When using some of the popular, highly reactive sulfides and Li metal (which is also highly reactive toward e.g. water) in an ASSB cell – then this cell is not for sure safer than one containing some (flamable) liquid electrolyte.
Hi 🙂
thanks for your comment. The points you mentioned helped me a lot
Joanna